From the desk of AHF’s Chief Medical Officer
Hi, I’m Dr. Wohlfeiler, the Chief Medical Officer for AIDS Healthcare Foundation in the United States. The COVID-19 vaccine rollout is underway across the nation. Every AHF healthcare center and pharmacy registered early on to be vaccine administration sites. Some of our healthcare centers have already received vaccines and administered them to patients and staff who meet the local eligibility requirements.
Right now, we’re vaccinating people 65 and over, frontline healthcare workers and, where state laws allow, people with HIV. We expect to see more eligibility tiers open up as the supply chain rapidly expands.
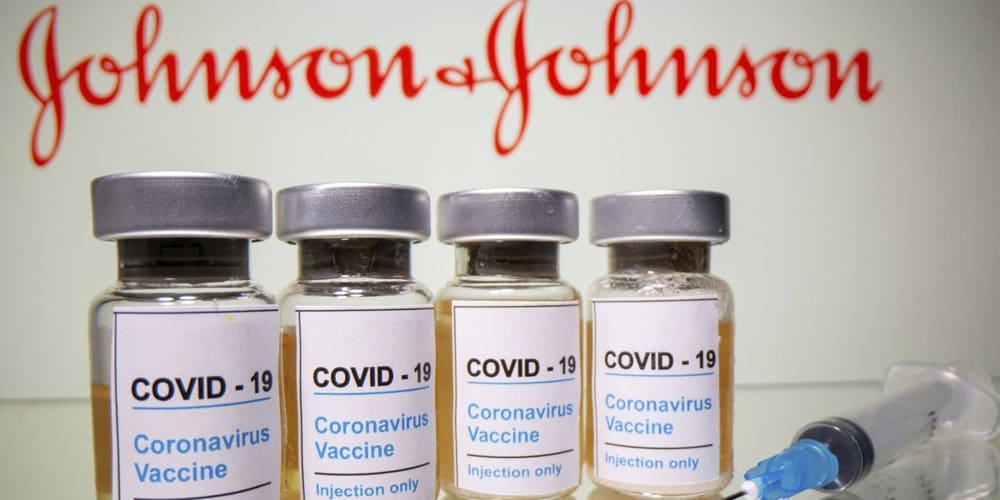
We expect supply to ramp up because a new COVID vaccine is now available. The new vaccine is from Johnson & Johnson and unlike the other vaccines, it only needs one dose and doesn’t require refrigeration. So, to summarize, there are now 3 vaccines available in the U.S. - the 2-dose Pfizer and Moderna vaccines and the new single-dose J&J vaccine.
Providers like AHF can’t choose which of the approved vaccines they receive. While the effectiveness of each vaccine is different, it’s our view as well as the view of the CDC that staff and patients should take whichever vaccine they can access earliest. Each of the vaccines will provide protection against the virus. And the more of us who have immunity, the safer we will be. So, please do your part and get vaccinated.
Michael Wohlfeiler, JD, MD
Chief Medical Officer

Most side effects are mild – like arm soreness or tiredness. Some people have symptoms that feel like the flu – chills, headaches, but these only generally last a day and can be treated with Tylenol or acetaminophen and rest. If you have these symptoms – you do not have COVID! Rather, they are a sign that your body is doing its job in developing antibodies to protect you.



I have HIV. Should I get the vaccine?
Yes. Being HIV-positive should not stop you from getting the COVID-19 vaccine. The only people who should not receive the vaccine are those who have a history of severe allergic reactions (anaphylaxis). It is important for people with HIV to be vaccinated because HIV might put them at an increased risk for severe illness if they get COVID-19.
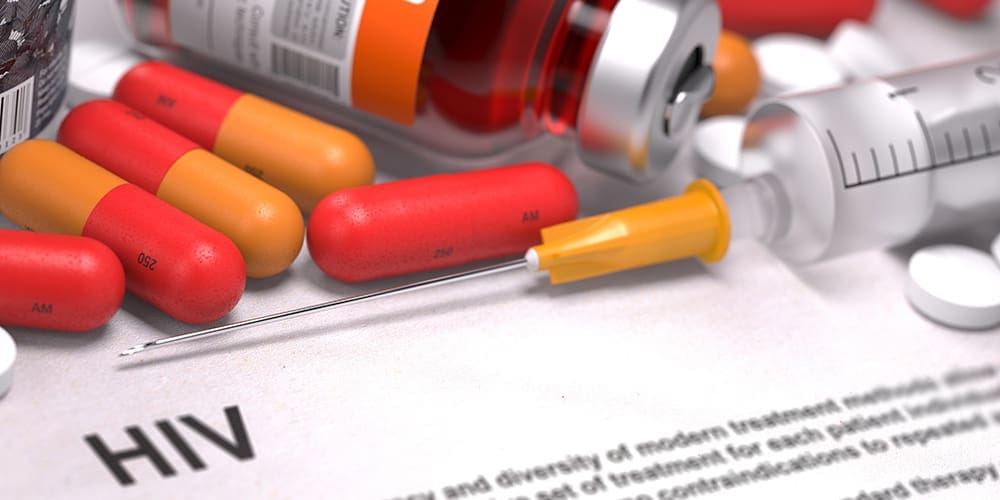
We believe that the vaccine will be effective and result in high levels of protection against COVID-19 in HIV-positive persons, especially if they are stable on antiretroviral therapy. If your T-cell count is less than 200, if your HIV is not under control or if you have other questions or concerns, you should speak with your medical provider prior to receiving the vaccine.
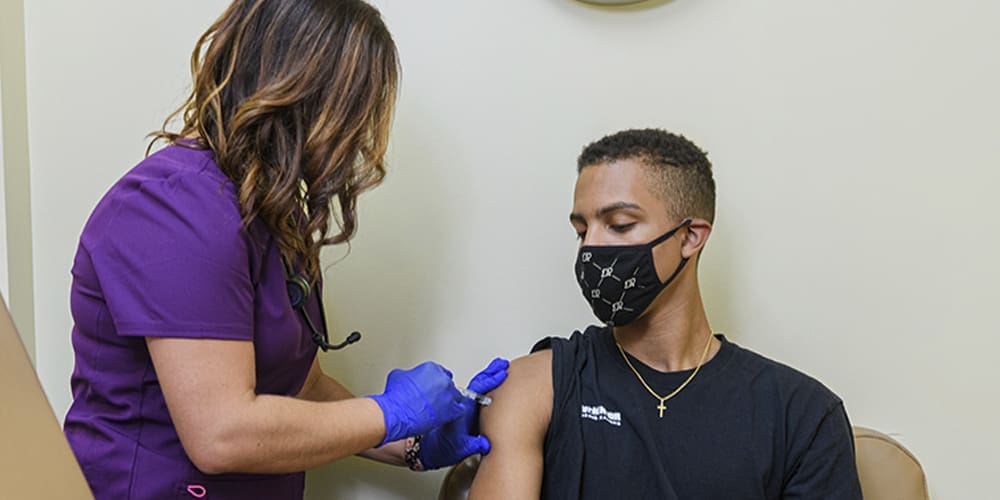
Please get the vaccine as soon as possible, from any trusted healthcare provider – whether AHF or otherwise. The sooner you get the vaccine, the sooner you’ll be protected.

As soon as AHF has the vaccine, we will start contacting patients to receive it. We’ll follow state and local guidelines. Right now, the first group will be people over a certain age (65 or 75) and people with serious health conditions. As state and local governments allow more people to get the vaccine, we will contact patients in these other groups.

Please do not wait if you can get the vaccine from another trusted healthcare provider. As soon as AHF has the vaccine, we will be offering it to pharmacy clients who according to state and local guidelines. We will be announcing when we have the vaccine and who is eligible by text messages, phone calls, and through our website.

How do we know the vaccination is safe?
Safety is our top priority at AHF. We're confident that the Food and Drug Administration (FDA) and the US Centers for Disease Control and Prevention (CDC) have ensured the safety of the COVID-19 vaccines. The U.S. vaccine safety system ensures all vaccines are as safe as possible.
After a vaccine is approved for use, there are many vaccine safety monitoring systems in place to watch for side effects that may not have been seen during clinical trials. If an unexpected symptom arises, experts quickly study it to decide whether it is a real safety concern.


I’m worried about the side effects. What are they?
Side effects include sore arm, mild fever, tiredness, headache and muscle aches. If you have any serious side effect, let your provider know right away. In rare cases, a few people have had a serious allergic reaction. If you have allergies, talk to your provider before getting the vaccine. At AHF, we have equipment and medicines on site to counteract an allergic reaction.


I hear that I need to have two shots - Do I really need two?

